教育及工作经历
2024–现在 kaiyun开云官方网站,特聘研究员
2023–2023 多特蒙德工业大学,Walter Benjamin博士后研究员,合作导师:Guido Clever教授
2019–2023 剑桥大学,Walter Benjamin博士后研究员,合作导师:Jonathan Nitschke教授
2015–2019 柏林工业大学,博士,导师:Martin Oestreich教授
2012–1015 上海大学,硕士,导师:龚和贵 教授
2008–2012 河南大学,学士,导师:史峰 教授
主要研究方向
1. 配位超分子化学:深入研究多层次手性配位超分子骨架的精准高效构建,探索手性产生、传递、放大和调控的机制与规律,为开发新型手性功能超分子组装体和材料提供有效方法和启示。
2. 金属有机催化:围绕新型高效手性配体及金属催化剂的高效合成,揭示手性配体骨架、配位原子、非共价相互作用、金属价态、自旋态以及受限环境等因素对催化剂性能的影响规律,为创制具有高活性和低价格的手性金属催化剂提供思路和方法。
依托于kaiyun开云官方网站金属有机络合物研究所,我们的课题组成立于2024年1月。我们诚挚邀请博士后、博士、硕士、研究助理以及联合培养员工加入。同时,欢迎本科生参与科研项目和毕业设计研究,您可以直接前往kaiyun开云官方网站金属有机络合物研究所办公室或发邮件咨询。
主要工作业绩
主要围绕第一过渡系金属在不同领域应用的系统性研究。通过实验论证和理论计算相结合,对第一过渡系金属形成的金属络合物和金属有机中间体进行深入研究,包括探究其氧化还原特性、亲核性、酸碱性、配位构型、自旋态等,在揭示其结构和活性的基础之上,设计并开发一系列绿色高效催化反应和手性金属有机笼。围绕上述研究工作,共计发表论文23篇,其中第一作者和通讯作者(含共同)15篇,包括 J. Am. Chem. Soc.(3篇)、Angew. Chem. Int. Ed.(5篇)、ACS Cent. Sci.(1篇)、Chem. Soc. Rev.(1篇)等。
主要奖励及荣誉
2024 开云全站中国有限公司双百B人才计划,开云全站中国有限公司
2019 Walter Benjamin博士后项目,德国科学基金会(DFG)
2019 最优等博士学位Summa Cum Laude,柏林工业大学
2015 研究生国家奖学金,上海大学
2015 光华奖学金,上海大学
代表性成果
1. W. Xue, L. Pesce, B. Adinarayana, T. K. Ronson, K. Wu, D. Zhang, N. Vanthuyne, T. Brotin, A. Martinez, G. M. Pavan, J. R. Nitschke. Subtle Stereochemical Effects Influence Binding and Purification Abilities of an FeII4L4 Cage. J. Am. Chem. Soc. 2023, 145, 5570–5575.
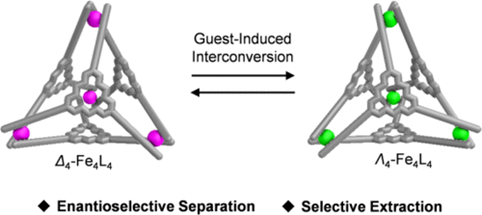
2. W. Xue, K. Wu, N. Ouyang, T. Brotin, J. R. Nitschke. Allosterically Regulated Guest Binding Determines Framework Symmetry for an FeII4L4 Cage. Angew. Chem. Int. Ed. 2023, 62, e202301319.

3. W. Xue, T. K. Ronson, Z. Lu, J. R. Nitschke. Solvent Drives Switching between Λ and Δ Metal Center Stereochemistry of M8L6 Cubic Cages. J. Am. Chem. Soc. 2022, 144, 6136–6142.

4. W. Xue,* X. Jia, X. Wang, X. Tao, Z. Yin, H. Gong*. Nickel-Catalyzed Formation of Quaternary Carbon Centers Using Tertiary Alkyl Electrophiles. Chem. Soc. Rev. 2021, 50, 4162–4184.
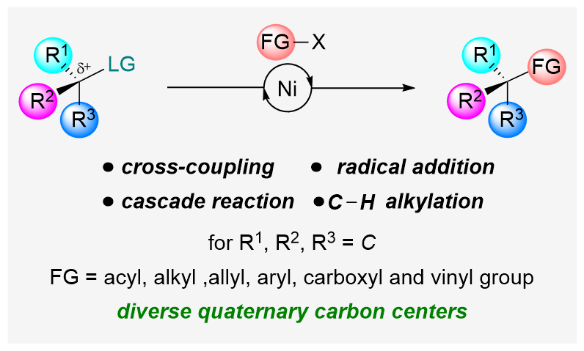
5. W. Xue, M. Oestreich. Beyond Carbon: Enantioselective and Enantiospecific Reactions with Catalytically Generated Boryl- and Silylcopper Intermediates. ACS Cent. Sci. 2020, 6, 1070–1081.

6. W. Xue,‡ W. Mao,‡ L, Zhang, M. Oestreich. Mechanistic Dichotomy of Magnesium- and Zinc-Based Germanium Nucleophiles in the C(sp3)–Ge Cross-Coupling with Alkyl Electrophiles. Angew. Chem. Int. Ed. 2019, 58, 6440–6443.

7. W. Mao,‡ W. Xue,‡ E. Irran, M. Oestreich. Copper-Catalyzed Regio- and Enantioselective Addition of Silicon Grignard Reagents to Alkenes Activated by Azaaryl Groups. Angew. Chem. Int. Ed. 2019, 58, 10723–10726. (Highlighted as Hot Paper; Synfacts 2019, 15, 1024).

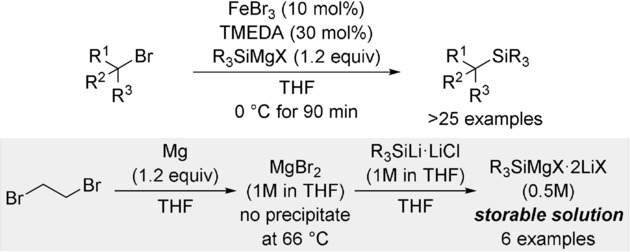
8. W. Xue, R. Shishido, M. Oestreich. Bench-Stable Stock Solutions of Silicon Grignard Reagents: Application to Iron- and Cobalt-Catalyzed Radical C(sp3)–Si Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2018, 57, 12141–12145. (Highlighted as VIP Paper; Synfacts 2018, 14, 1174; Org. Process Res. Dev. 2018, 22 ,1687)
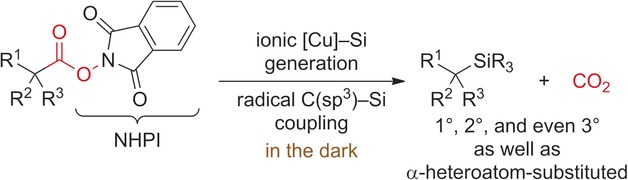
9. W. Xue, M. Oestreich. Copper-Catalyzed Decarboxylative Radical Silylation of Redox-Active Aliphatic Carboxylic Acid Derivatives. Angew. Chem. Int. Ed. 2017, 56, 11649–11652.
10. W. Xue, Z.-W. Qu. S. Grimme, M. Oestreich. Copper-Catalyzed Cross-Coupling of Silicon Pronucleophiles with Unactivated Alkyl Electrophiles Coupled with Radical Cyclization. J. Am. Chem. Soc. 2016, 138, 14222–14225.
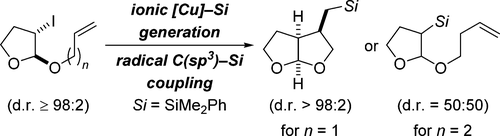
11. W. Xue, M. Oestreich. Silicon Grignard Reagents as Nucleophiles in Transition-Metal-Catalyzed Allylic Substitution. Synthesis 2019, 51, 233–239.
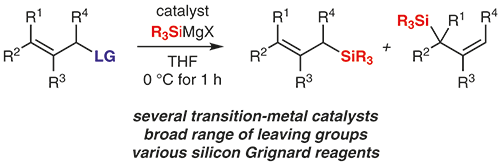
12. W. Xue,‡ H. Xu,‡ Z. Liang, H. Gong. Nickel-Catalyzed Reductive Cyclization of Alkyl Dihalides.Org. Lett. 2014, 16, 4984–4987. (Highlighted by Synfacts, 2015, 11, 0076)

13. Z. Liang,‡ W. Xue,‡ K. Lin, H. Gong. Nickel-Catalyzed Reductive Reductive Methylation of Alkyl Halides and Acid Chlorides with Methyl p-Tosylate. Org. Lett. 2014, 16, 5620–5623.
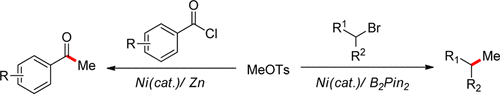
14. Z. Zhao, Y. Gong, W. Tong, W. Xue*, H. Gong.* Ni-Catalyzed Cross-Electrophile Coupling of Aryl Triflates withThiocarbonates via C–O/C–O Bond Cleavage. Org. Lett. 2021, 23,2158–2163.
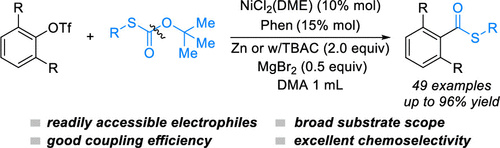
15. X. Tao, K. Yao,* W. Xue.* Ni-Catalyzed Cross-Electrophile Coupling of α-Hydroxy Carbonyl Compound-Derived Oxalates with Vinyl Triflates. Tetrahedron Lett. 2021, 73, 153129.
16. H. Zhu, L. Pesce, R. Chowdhury, W. Xue, K. Wu, T. K. Ronson, R. H. Friend, G. M. Pavan, J. R. Nitschke. Stereocontrolled Self-Assembly of a Helicate-Bridged CuI12L4 Cage That Emits Circularly Polarized Light. J. Am. Chem. Soc. 2024, 10.1021/jacs.3c11321.
17. K. Wu, T. K. Ronson, L. Goh, W. Xue, A. W. Heard, P. Su, X. Li, M. Vinković, J. R. Nitschke. A Diverse Array of Large Capsules Transform in Response to Stimuli. J. Am. Chem. Soc. 2023, 145, 11356–11363
18. S. Bähr, W. Xue, M. Oestreich. C(sp3)–Si Cross-Coupling. ACS Catal. 2019, 9, 16–24.
19. M. Zheng, W. Xue, T. Xue, H. Gong. Ester Formation via Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Chloroformates. Org. Lett. 2016, 18, 6152–6155.
20. X. Wang, S. Wang, W. Xue, H. Gong. Nickel-Catalyzed Reductive Coupling of Aryl Bromides withTertiary Alkyl Halides. J. Am. Chem. Soc. 2015, 137, 11562–11565.
21. J. Gu, X. Wang, W. Xue, H. Gong. Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front. 2015, 2, 1411–1421. (ESI Highly Cited Paper)
22. P. Li, C. Wu, J. Zhao, Y. Li, W. Xue, F. Shi. One-Pot Synthesis of Dihydrobenzisoxazoles from Hydroxylamines, Acetylenedicarboxylates, and Arynes via in situ Generation of Nitrones. Can. J. Chem. 2013, 91, 43–50.
23. C. Wu, P. Li, Y, Fang, J, Zhao, W. Xue, Y. Li, R. C. Larock, F. Shi. Pd-Catalyzed Oxidative Coupling of Monosubstituted Sydnones and Terminal Alkynes. Tetrahedron Lett. 2011, 52, 3797–3801.
