Education
Ph.D. Organic Chemistry, Chiral Chemistry and Asymmetric Catalysis
Sichuan University, Chengdu, Sichuan, P.R. China, June 2009
Dissertation: Studies of Amino Amide Based Organometallic Complex and
Organocatalyst Design, Synthesis and Their Application in Asymmetric Catalyses
Thesis Advisor: Dr. Xiaoming Feng (Academician of Chinese Academy of Sciences)
B.S. Applied Chemistry
Sichuan University, Chengdu, Sichuan, P.R. China, June 2004
Dissertation: Asymmetric Synthesis of 2,5-Disubstituted Dihydropyranones
Thesis Advisor: Dr. Xiaoming Feng (Academician of Chinese Academy of Sciences)
Research Area of Specialty
Organic Synthetic Chemistry
Bioactive Molecular Synthesis
Biochemistry, Bioorthogonal Chemistry
Professional Experience
August 2014 – March 2015: |
Research Assistant Professor |
|
Department of Chemistry |
|
The State University of New York, University at Buffalo |
|
Principle Investigator: Dr. Qing Lin |
|
Conducts research on Photon-induced bioorthogonal chemistry, Genetic or metabolic incorporation of unnatural amino acids into target proteins in mammalian cells; Mechanisms of 1,3-dipolar cycloaddition reaction and its effect on improving the bioorthogonality. The use of chemical tools to study conformational dynamics of the class B G-protein coupled receptors in living cells |
August 2009 – August 2014: |
Biochemistry Research Associate |
|
Postdoctoral Associate |
|
Department of Chemistry |
|
Conducts research on photon induced 1,3-dipolar cycloaddition reaction between tetrazole and alkene; Photon induced bioorthogonal chemistry, Genetic or metabolic incorporation of unnatural amino acids into target proteins in mammalian cells; Mechanisms of 1,3-dipolar cycloaddition reaction and its effect on improving the bioorthogonality. |
|
|
September 2004 - June 2009: |
Graduate Student for Ph. D. |
|
College of Chemistry Sichuan University |
|
Conducted research on the asymmetric catalysis based on novel design of amino amide derived chiral catalyst; application of chirotechnology in total synthesis of natural products; optimization of asymmetric organocatalysis on gram-scale; mechanism study of chiral bifunctional guanidine catalyst; design of chiral N, N’-dioxide-Indium(III) complex and its application in Hetero- Diels-Alder reaction. |
Honors and Award:
1. 2008 Special Top Award, the Baosteel Education Foundation 2008,Sichuan University.
2. 2008 First Rank Award, the Creative Talent Award, Sichuan University.
3. 2005 Second Rank Award, the Scholarship for Outstanding Graduate Students, Sichuan University.
4.2002 Second Rank Award, the Fourth Scientific and Technological Festival for Students, Animation Designation, Sichuan University.
Publications:
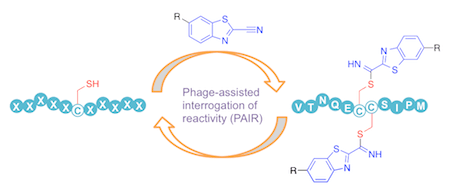
1.Co-author Research publication:Carlo P. Ramil,Peng An,Zhipeng Yu,and Qing Lin*, titled "Sequence-specific 2-cyanobenzothiazole ligation "J. Am. Chem. Soc.,J. Am. Chem. Soc.,2016,138, 5499–5502.
2.First author Research publication: Zhipeng Yuand Qing Lin*, titled "Design of Spiro[2.3]hex-1-ene, a Genetically Encodable Double-Strained Alkene for Superfast Photoclick Chemistry"J. Am. Chem. Soc.,2014, 136, 4153–4156.
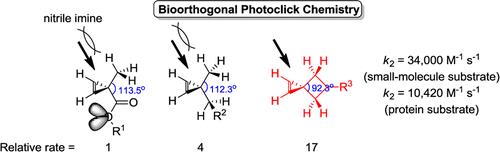
3.First author Research publication: Zhipeng Yu,Tymish Y. Ohulchanskyy, Peng An, Paras N. Prasad, and Qing Lin*, titled "Fluorogenic, Two-Photon-Triggered Photoclick Chemistry in Live Mammalian Cells"J. Am. Chem. Soc.,2013,135, 16766–16769.
4.First author Research publication: Zhipeng Yu,Yanchao Pan, Zhiyong Wang, Jiangyun Wang,* Qing Lin*, titled "Genetically Encoded Cyclopropene Directs Rapid, Photoclick-Chemistry-Mediated Protein Labeling in Mammalian Cells"Angew. Chem. Int. Ed.2012,51, 10600-10604.
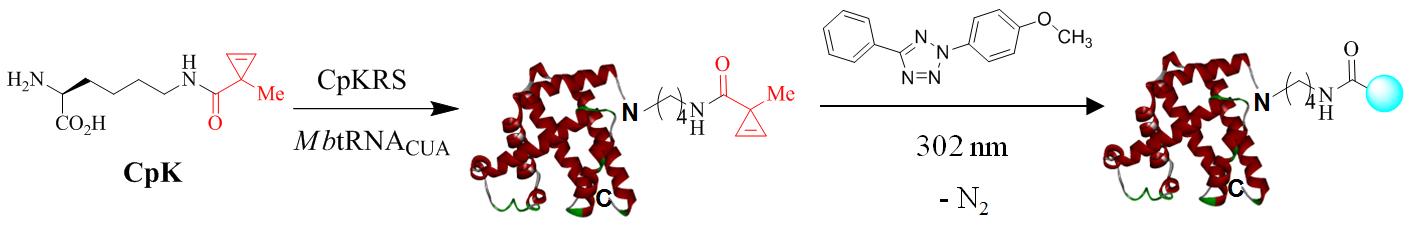
5.First author Research publication: Zhipeng Yu, Lok Yin Ho, and Qing Lin*, titled "Rapid, Photoactivatable Turn-On Fluorescent Probes Based on an Intramolecular Photo click Reaction"J. Am. Chem. Soc.,2011,133, 11912–11915.
6.First author Research publication:Zhipeng Yu, Lok Yin Ho, Zhiyong Wang, Qing Lin*, titled "Discovery of new photoactivatable diaryltetrazoles for photoclick chemistry via ‘scaffold hopping’"Bioorg. Med. Chem. Lett.,2011,21, 5033-5036.
7.First author Research publication:Zhipeng Yu, Reyna K. V. Lim, Qing Lin*, titled "Synthesis of Macrocyclic Tetrazoles for Rapid Photoinduced Bioorthogonal 1,3-Dipolar Cycloaddition Reactions"Chem. Eur. J.2010,16, 13325-13329
8.Co-author Research publication:Peng An,Zhipeng Yuand Qing Lin*, titled "Design of oligothiophene-based tetrazoles for laser-triggered photoclick chemistry in living cells"Chem. Commun.,2013,49,9920-9922.
9.Co-author Research publication:Peng An,Zhipeng Yuand Qing Lin*, titled "Design and Synthesis of Laser-Activatable Tetrazoles for a Fast and Fluorogenic Red-Emitting 1,3-Dipolar Cycloaddition Reaction"Org. Lett.,2013, 15,5496–5499.
10.Co-author Research publication:Jiangyun Wang, Wei Zhang, Wenjiao Song, Yizhong Wang,Zhipeng Yu, Jiasong Li, Minhao Wu, Lin Wang, Jianye Zang, and Qing Lin*, titled "A Biosynthetic Route to Photoclick Chemistry on Proteins"J. Am. Chem. Soc.,2010,132, 14812–14818.
11.Co-author Research publication:Wenjiao Song, Yizhong Wang,Zhipeng Yu, Claudia I. Rivera Vera, Jun Qu, and Qing Lin*, titled "A Metabolic Alkene Reporter for Spatiotemporally Controlled Imaging of Newly Synthesized Proteins in Mammalian Cells"ACS Chem. Biol.,2010,5, 875–885 (Cover Article)
12.Co-author Research publication:Wenjiao Song,Zhipeng Yu,Michael M. Madden and Qing Lin*, titled "A bioorthogonal chemistry strategy for probing protein lipidation in live cells"Mol. BioSyst.,2010,6, 1576-1578.
13.First author Research publication:Zhipeng Yu, Xiaohua Liu, Lin Zhou, Lili Lin, Xiaoming Feng*, titled "Bifunctional Guanidine via an Amino Amide Skeleton for Asymmetric Michael Reactions of β-Ketoesters with Nitroolefins: A Concise Synthesis of Bicyclicβ-Amino Acids"Angew. Chem. Int. Ed.2009,48, 5195-5198.
14.Co-author Research publication:Shao-Liang Zheng, Yizhong Wang,Zhipeng Yu, Qing Lin*, and Philip Coppens*, titled "Direct Observation of a Photoinduced Nonstabilized Nitrile Imine Structure in the Solid State"J. Am. Chem. Soc.2009,131, 18036-18037.
15.First author Research publication:Zhipeng Yu,Xiaohua Liu, Zhenhua Dong, Mingsheng Xie, and Xiaoming Feng*, titled "AnN,N’-Dioxide/In(OTf)3Catalyst for the Asymmetric Hetero-Diels-Alder Reaction Between Danishefsky’s Dienes and Aldehydes: Application in the Total Synthesis of Triketide".Angew. Chem. Int. Ed.2008,47, 1308-1311.
16.Co-author Research publication:Bo Gao,Zhipeng Yu, Zhengyan Fu and Xiaoming Feng*, titled "A Convenient and Versatile Approach to 2,3-dihydro-4H-pyran-4-ones via Tandem Aldol Reaction-conjugate Addition",Tetrahedron Letters2006,47, 1537-1539.
17.Co-author Research publication:Bo Gao, Zhengyan Fu,Zhipeng Yu, Lan Yu, Yaozong Huang and Xiaoming Feng*, titled "Highly enantioselective hetero-Diels–Alder reaction betweentrans-1-methoxy-2-methyl-3-trimethyl-siloxybuta-1,3-diene and aldehydes catalyzed by (R)- BINOL-Ti(IV) complex",Tetrahedron2005,61, 5822-5830.
18.Co-author Research publication:Ke Cheng, Baiqing Zeng,Zhipeng Yu, Bo Gao, Xiaoming Feng*, titled "A Mild and Efficient Synthesis of 2,5-disubstituted 2,3-Dihydro-4-pyridones Catalyzed by Yb(OTf)3",Synlett.2005,1018-1020.
19.Co-author Research publication:Zhengyan Fu, Bo Gao,Zhipeng Yu, Lan Yu, Yaozhong Huang, Xiaoming Feng,* Guolin Zhang,* titled "An Efficient and Enantioselective Approach to 2,5-Disubstituted Dihyropyrones",Synlett.2004, 1772-1775.
Research achievement and interest
The design and development of multifunctional chiral organocatalysts is of great importance: single catalyst molecule possessing two or more promoting functionalities via different activation sites is more robust and specific in chiral induction pocket. Therefore, the reactivity and selectivity of the organocatalyst can be finely tuned through combining multi-valent moiety of the simple catalytical core. Recently, I have turned my attention on the organocatalyst design under the supervision of Prof. Xiaoming Feng. We developed a novel bifunctional guanidine organocatalyst via lithium amino amide to carbodiimide. To assess its performance, we also have presented an example of the asymmetric Michael addition ofβ-ketoesters to nitroolefins by introducing the novel organocatalytical guanidine as catalyst. Driven by the aim of developing novel catalyst, I always dedicate myself in the innovation of catalytic framework which the original source to promote asymmetric chemistry. In the near future, extension of my profession in biochemistry aspect would also be my favorite. To carve out my career, I would also like to exert my strong point into facilitating bioactive molecule synthesis, chiral material chemistry, molecular biology, especially interdisciplinary academic research. I have confidence that the combination of various technique and knowledge would bring forth some fascinating research outcomes.
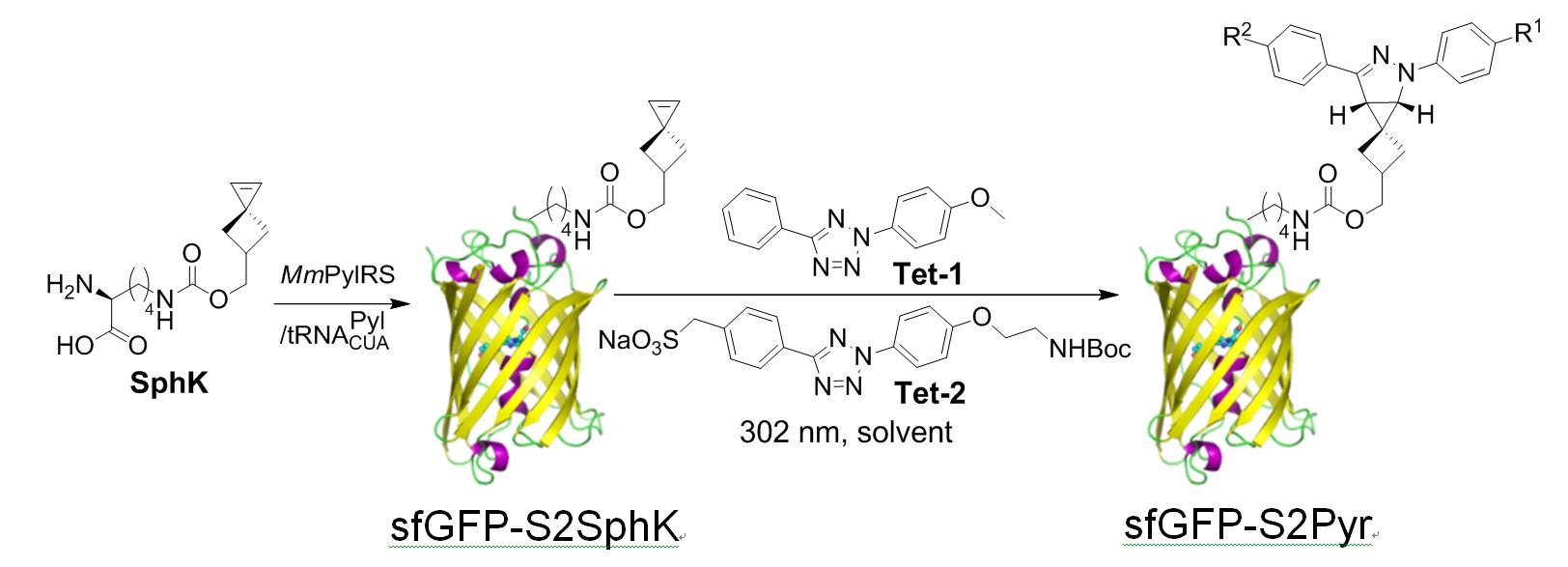
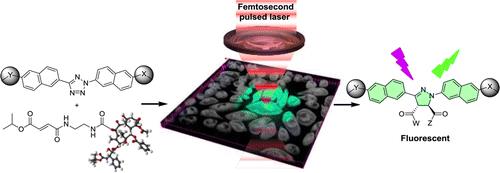
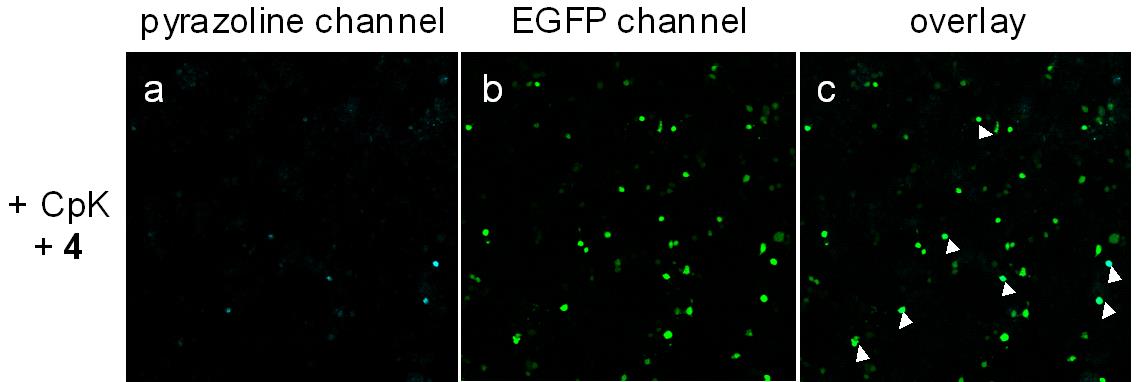
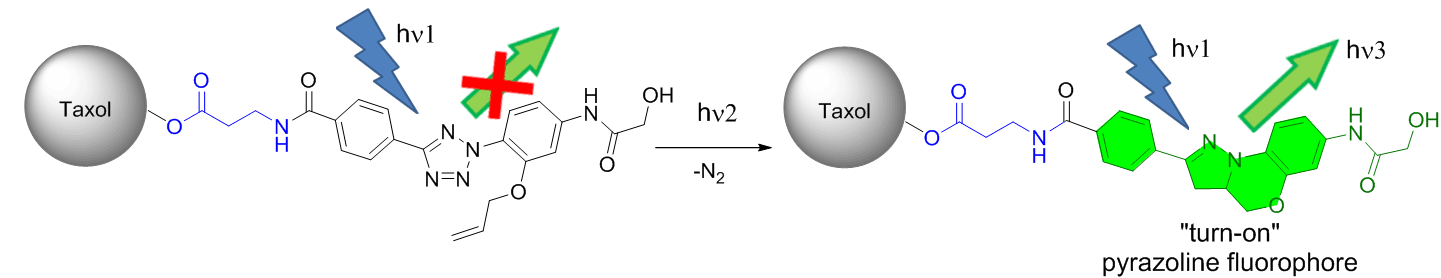
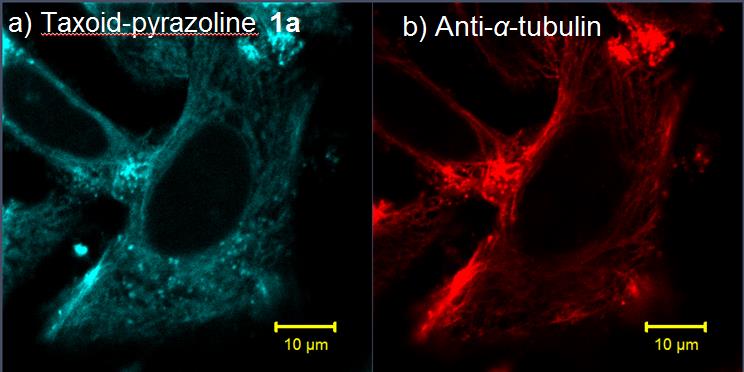
Working on the synthesis of highly reactive and selective 1,3-dipole precursor for bioorthogonal ‘photon-click’ reactions is our interest because ultra-fast reaction is greatly beneficial for biological studies. By employing a ‘scaffold hopping’ strategy, as we reported recently, it should be possible to further improve the photo activation efficiency, for example, by increasing absorption at 405-nm through the use of other aromatic scaffolds, and obtain turn-on pyrazoline fluorophores with increased brightness and photo-stability. Furthermore, we have demonstrated a new strategy for designing turn-on fluorescent probes via intramolecular ‘photo-click’ reaction, in which a protein-binding molecule is tagged with a photoactivatable, alkene-appended tetrazole. People have taken advantages of this strategy to photo-controlled Hydrogel solidification and liquidation for 3D-cell culture. Recently by fine tuning the tetrazole structure and introducing hetero aromatic ring, we are broadening the photo-click chemistry to specifically label genetic encoded UAA (non-canonical amino acids) labeled protein and biomolecule in cell triggered by femtosecond laser pulse (two-photon ‘photo-click’ chemistry). It’s of great interest to synthesize tetrazole molecules of unique property to exert their advantages in probing or manipulating the biological process in vitro and in vivo with spatial-temporal precision. Previously we have reported the synthesis of a stable and genetically encodable 3,3-disubstituted cyclopropene for photo-click reaction by use of strain, a classic concept in organic chemistry, to accelerate bioorthogonal reactions. We were able to reach 58 M-1s-1 second order reaction kinetics. Very recently, by fusing to strained small ring together, we synthesized a second generation cyclopropene, Spiro[2.3]hex-1-ene (Sph), for photo-click chemistry. Since more reactive yet stable Sph-L-lysine was used for genetically incorporation into protein, we were able to achieve fast reaction kinetics (k 2 up to 10000 M?1s?1) in the photo-click chemistry and a?orded rapid (<10 s) bioorthogonal protein labeling.
